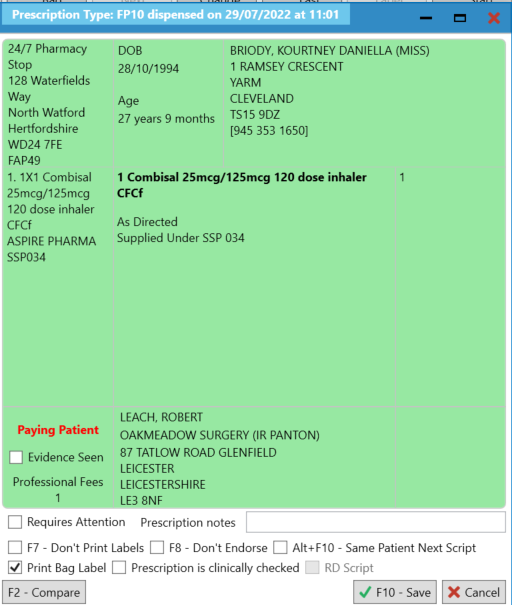
Endorsement Guidance for SSP034 Combisal® 125mcg/25mcg inhalers
NHSBSA have announced a substitution SSP for Combisal (Fluticasone 125microgram / Salmeterol 25microgram) pressurised metered dose inhaler (pMDI). If the inhaler cannot be supplier another Fluticasone 125microgram / Salmeterol 25microgram pMDI can be supplied instead
If a patient uses a spacer with their inhaler, the pharmacist should attempt to provide a substituted inhaler compatible with the patient’s spacer. If this is not possible, the pharmacist should supply the patient with a spacer compatible with the inhaler free of charge and claim reimbursement when claiming for the SSP.
NHSBSA have confirmed that NCSO can be used to assist in endorsing two items against one prescribed item. SSP for inhaler and NCSO for spacer.
Please follow this guidance to ensure the prescription is endorsed correctly in ProScript Connect:
- In ProScript Connect, process the prescription as normal and remain in the PMR.
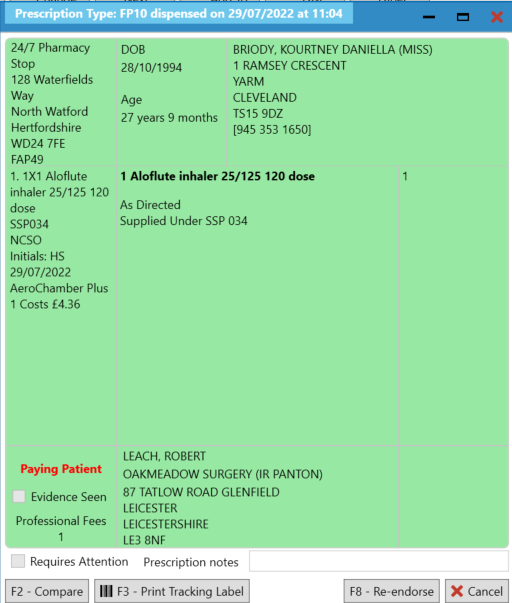
- We recommend that at this stage, you add “Supplied under SSP [SSP reference number]” so that this is reflected on the label. This is a mandatory requirement therefore ensure this is added as per your business processes.
- Highlight the item in the prescription container and select [Additional Endorsements]. The Additional Endorsements window may be invoked during dispensing, depending on the setting of the Auto Endorse Short Supply application setting.
- Scroll down the list and select “Serious Shortage Protocol”. In the field, you will need to enter the SSP reference number. Ensure that you add this as a three-digit value. For example, SSP034 would be entered in as 034.
- Select [F8 – Add Item] and then [F10 – Save].
- Save and complete the prescription. You may wish to select [F7 – Don’t Print Labels] if the only item you are endorsing is the SSP item.
- You will be prompted with the following message, which you can print to a dispensing label to aid the clinical and accuracy check processes:
- You will then be prompted with the following message:
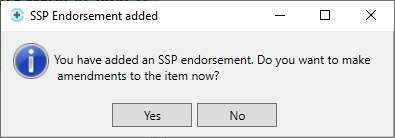
- We recommend that you make the changes to the item at this stage to prevent a claim being submitted with the original item; if this is done, the NHS BSA may require further information from you to support the claim.
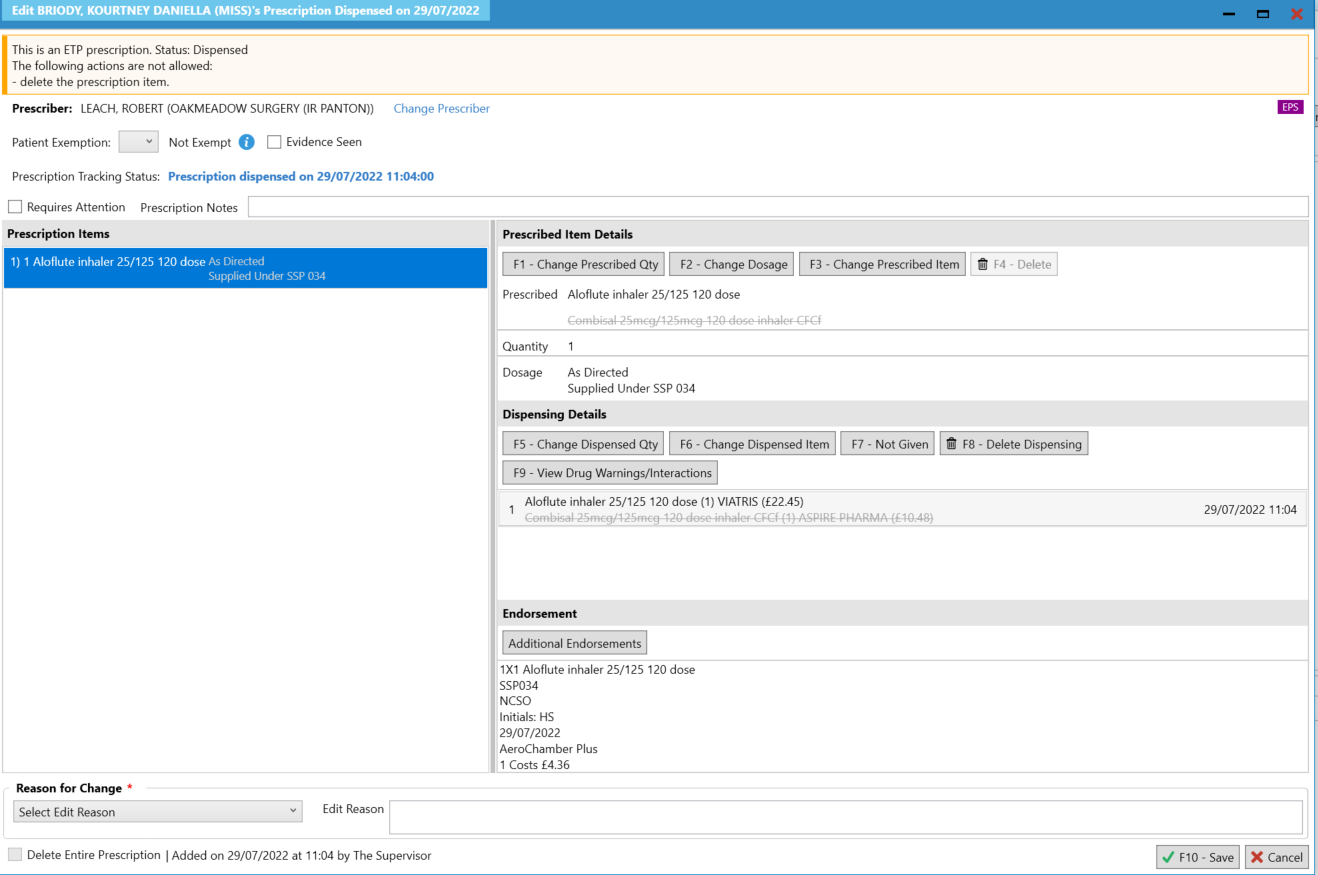
- Upon selecting [Yes], you will be navigated to the Edit Script screen for the prescription you have just dispensed.
- On the Edit Script screen, amend the supplied item using the [F3 – Change Prescribed Item] function. Select the inhaler that will be dispensed to the patient. Please note when the prescribed item is changed the dispensed item will also be amended.
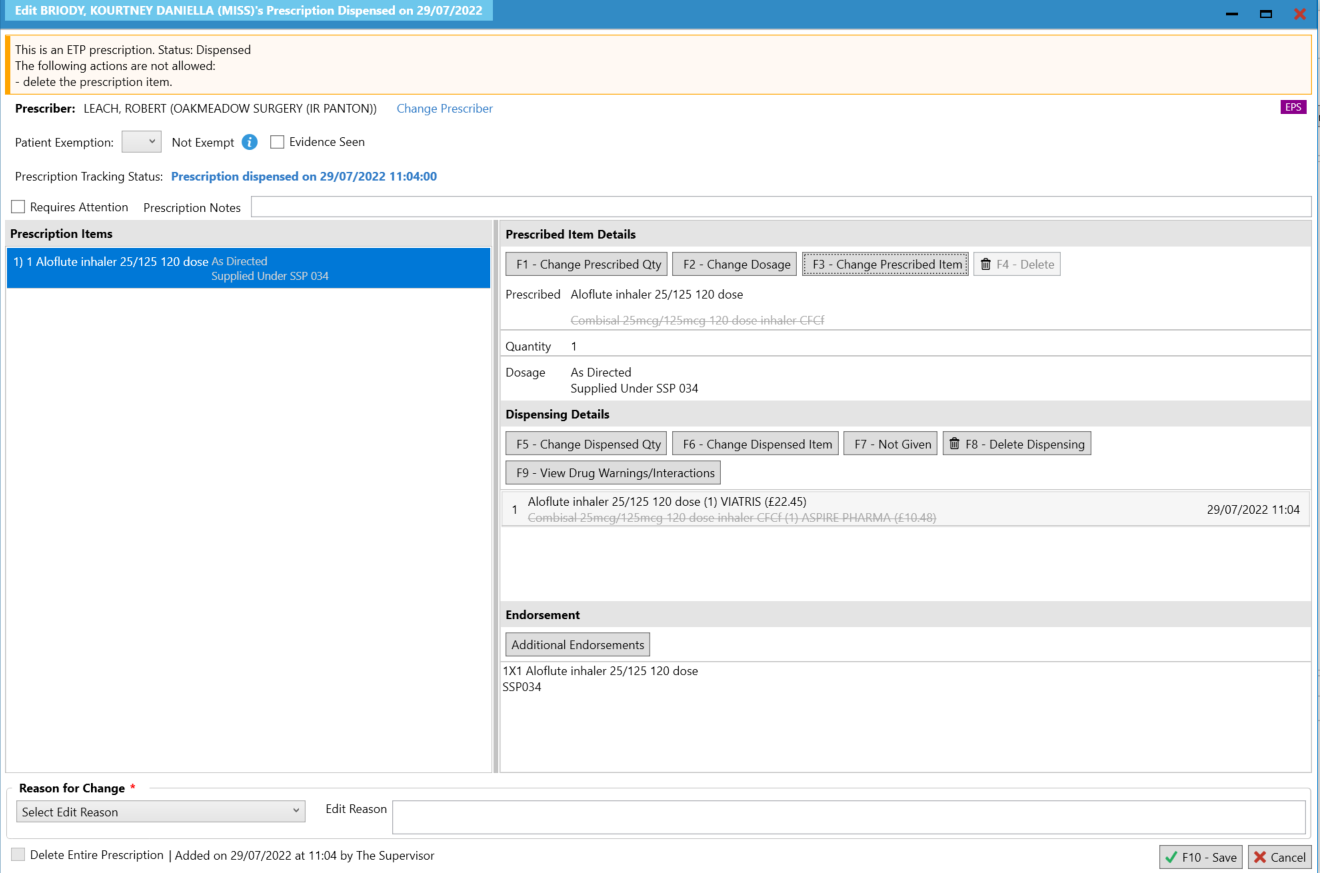
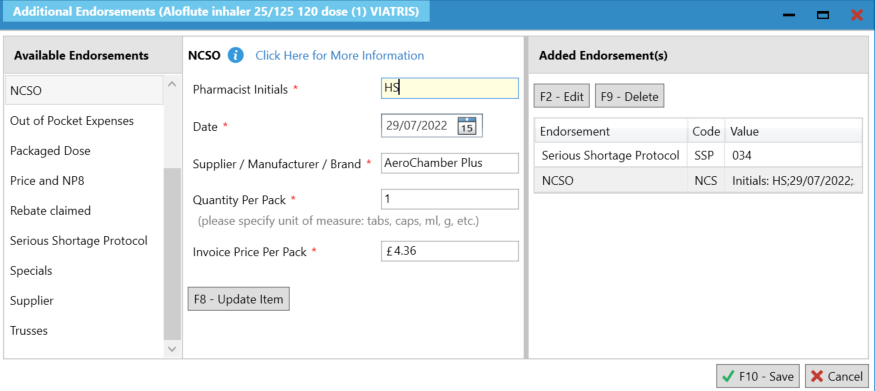
- If a spacer has been provided this can be endorsed using the NCSO endorsement. On the Edit Script screen, select Additional Endorsements and navigate to NCSO. The following information will be required:
- The Supplier/Manufacturer/ Brand field can be used to specify the details of the product supplied under SSP. This should include the drug name, strength, formulation and brand or supplier if required.
- Pack size (where multiple pack sizes are available)
- Invoice price (where there is no list price held by NHSBSA)
- Select reason for change then F10-Save.When the item is claimed the information that was entered on the Edit Script screen will be sent for reimbursement.
Updated 19/08/2022 JHG
