Sodium Valproate – Special Container dispensing
Following the change in special container status to whole pack dispensing for all licensed medicines containing sodium valproate, valproic acid and semi sodium valproate, sites have reported issues in dispensing certain sodium valproate prescriptions as they are unable to obtain stock of all pack sizes.
This wasn’t an issue previously as these items were sub pack special containers in strips of 10 which could be given from either pack size.
If there are multiple pack sizes of a special container, ProScript Connect will use the current Drug Tariff and CPE rules and calculate the nearest quantity based on any combination of pack sizes and not the nearest number of full packs of a single pack size. We have been in contact with NHSBSA and CPE and we now have confirmation that for sodium valproate they will reimburse on the nearest quantity of a single pack size as long as that single pack size is endorsed.
The examples we have been provided are:
One pack of 100 and 2 x 30 tablets dispensed
Prescribed: Sodium valproate 200mg gastro-resistant tablets x 168 tablets
Endorsed: no endorsement
Reimbursed: 1 x 100 pack and 2 x 30 pack
or
Prescribed: Sodium valproate 200mg gastro-resistant tablets x 168 tablets
Endorsed: 100/100 and 60/30
Reimbursed: 1 x 100 pack and 2 x 30 pack
Six packs of 30 dispensed
Prescribed: Sodium valproate 200mg gastro-resistant tablets x 168 tablets
Endorsed: 180/30
Reimbursed: 6 x 30 pack
Two packs of 100 tablets dispensed
Prescribed: Sodium valproate 200mg gastro-resistant tablets x 168 tablets
Endorsed: 200/100
Reimbursed: 2 x 100 pack
To help when processing prescriptions for Sodium valproate 200mg gastro-resistant tablets or Sodium valproate 500mg gastro-resistant tablets, we have created multiple families in ProScript Connect to allow the Special Container functionality to work based on a single pack size.
When processing a prescription for either Sodium valproate 200mg gastro-resistant tablets or Sodium valproate 500mg gastro-resistant tablets you will now be able to select from either a mixed family (packs of both 30 and 100), a family with just packs of 30 or a family with just packs of 100.
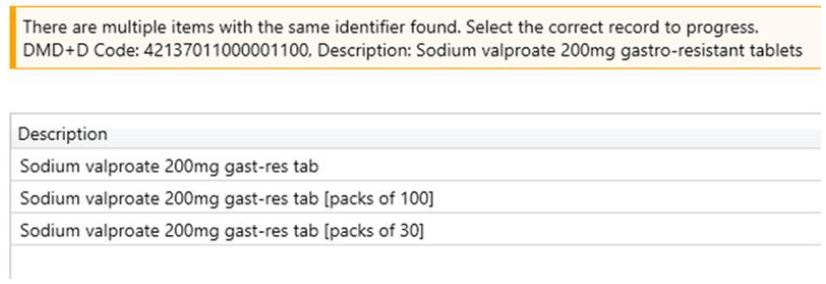
The special container rules will then only apply based on the pack sizes in that family.
For example, if a prescription for 168 tablets is received and the user selects the 30-pack size family. The special container rules will recognise this and allow the quantity to be rounded up to 180 to supply 6 packs of 30 tablets.
As soon as the discontinued pack size has been removed from part 8A of the Drug Tariff and only one pack size remains as a special container, these additional families will no longer be required and will be retired.
