NMS Expansion: September 2021
Background
From 1st September 2021, contractors can provide the New Medicines Service (NMS) to patients that are newly prescribed medicines for the following additional conditions:
- Acute coronary syndrome (ACS);
- Atrial fibrillation (AF);
- Coronary heart disease;
- Epilepsy;
- Glaucoma;
- Gout;
- Heart failure;
- Hypercholesterolaemia ;
- Long term risks of venous thromboembolism/embolism;
- Osteoporosis;
- Parkinson’s Disease;
- Stroke / transient ischemic attack; and
- Urinary incontinence and retention.
The NHSBSA have published an updated list of medicines that are suitable for NMS covering the above conditions.
Please note that the limit on the number of NMS that can be conducted has increased from 0.5% to 1% of the monthly prescription volume and additional bandings will also be included.
EMIS are reviewing the updated service specification and will be updating the NMS module in PSC in due course.
Drug Triggers in ProScript Connect
The EMIS Community Pharmacy Informatics team will be updating the drugs database to flag these items as eligible for an NMS for our next scheduled PKBRuntime update. This will update will be available from Friday 10th September 2021.
You will be prompted to conduct an NMS when dispensing one of these new medications at the end of the dispensing process, as per the existing target groups.
Conducting an NMS in ProScript Connect
Once the latest drugs database (PKBRuntime) has been applied to ProScript Connect (PSC), you will be triggered to carry out an NMS in the same manner.
- For example, if Allopurinol 100mg tablets was newly prescribed to treat gout, you would be prompted as follows in PSC:
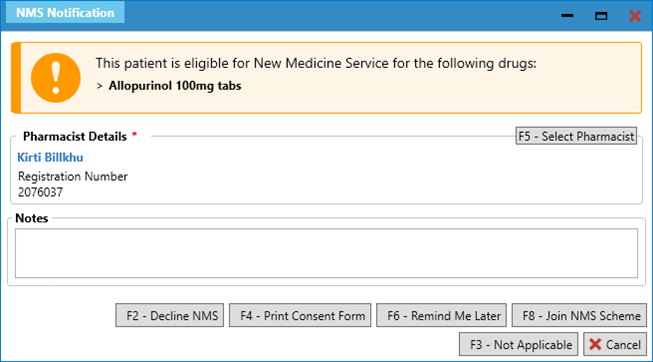
- Upon selecting [F8 – Join NMS Scheme], you will be navigated to the NMS – Patient Engagement tab, which you should complete as normal.
- Currently, the ‘Condition(s) / Therapy Areas’ will not reflect the additional conditions.
- If the NMS is for:
- One medication: we recommend that you select one of these check boxes (as this section is mandatory) and enter detail into the Notes field to reflect the intended condition as well as the condition you have selected. This will later be used to support you in updating the quarterly report figures.
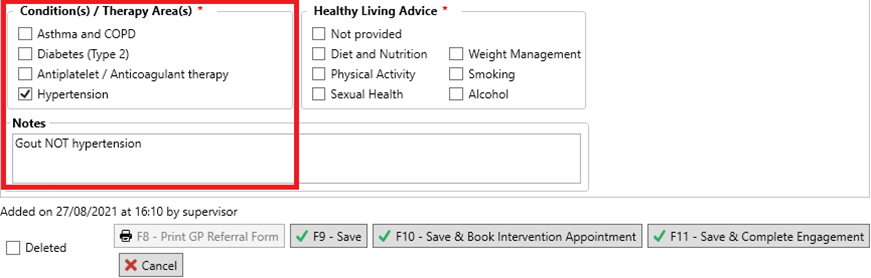
- One medication: we recommend that you select one of these check boxes (as this section is mandatory) and enter detail into the Notes field to reflect the intended condition as well as the condition you have selected. This will later be used to support you in updating the quarterly report figures.
- If the NMS is for:
- Multiple medications: we recommend that you select one of these checkboxes (as this section is mandatory) and enter detail into the Notes field to reflect the intended condition as well as the condition you have selected. This will later be used to support you in updating the quarterly report figures.
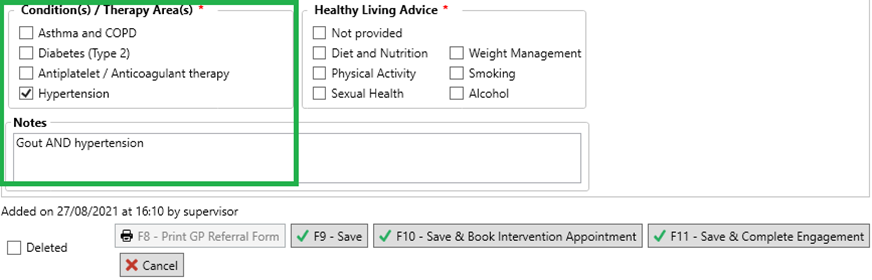
- Multiple medications: we recommend that you select one of these checkboxes (as this section is mandatory) and enter detail into the Notes field to reflect the intended condition as well as the condition you have selected. This will later be used to support you in updating the quarterly report figures.
- You then complete the NMS as normal.
For continuity you may wish to make a record of the condition/therapy area for all NMS in the notes section regardless of the box you select.
The existing antiplatelet/anticoagulant therapy group is now covered under multiple new therapy areas referencing the underlying condition or reason for prescribing. Therefore no NMS will fall under this group as of September 1st 2021. You may therefore wish to mark all NMS carried out under one of the new therapy groups under this removed group and use the Notes section to record the actual condition “new” therapy area.
Reporting in PSC
As the ‘Condition(s) / Therapy Areas’ will not currently reflect the additional conditions, the quarterly report figures will need to be amended in order to submit the claims under the correct condition areas.
- In the NMS Manager, filter your patients by a suitable date period and filter Stage to show “Completed Claimable”.
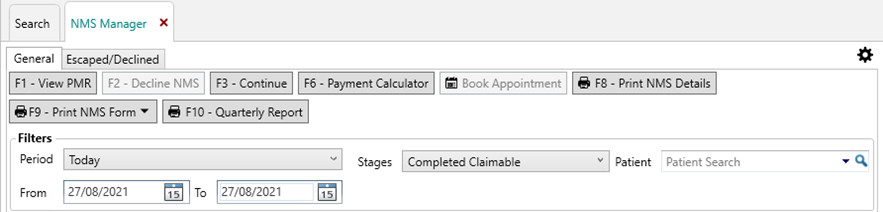
- Next, select the [F8 – Print NMS Details] button to generate a report of patients. This can be printed and hand-annotated to record their condition(s)/therapy area:
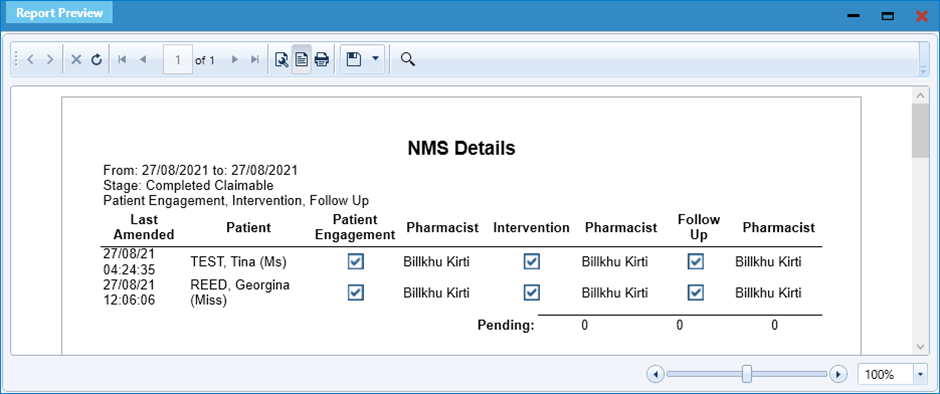
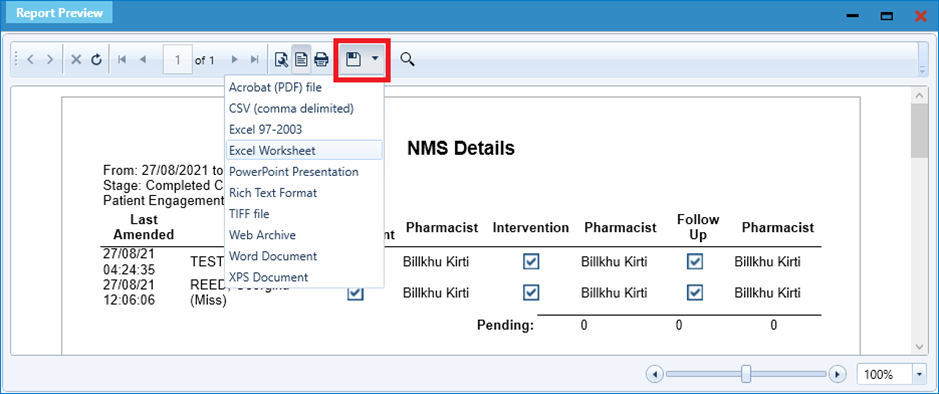
- If you are displayed with a report preview (as shown above), you can export these results to Excel by selecting the Save icon and selecting Excel Worksheet:
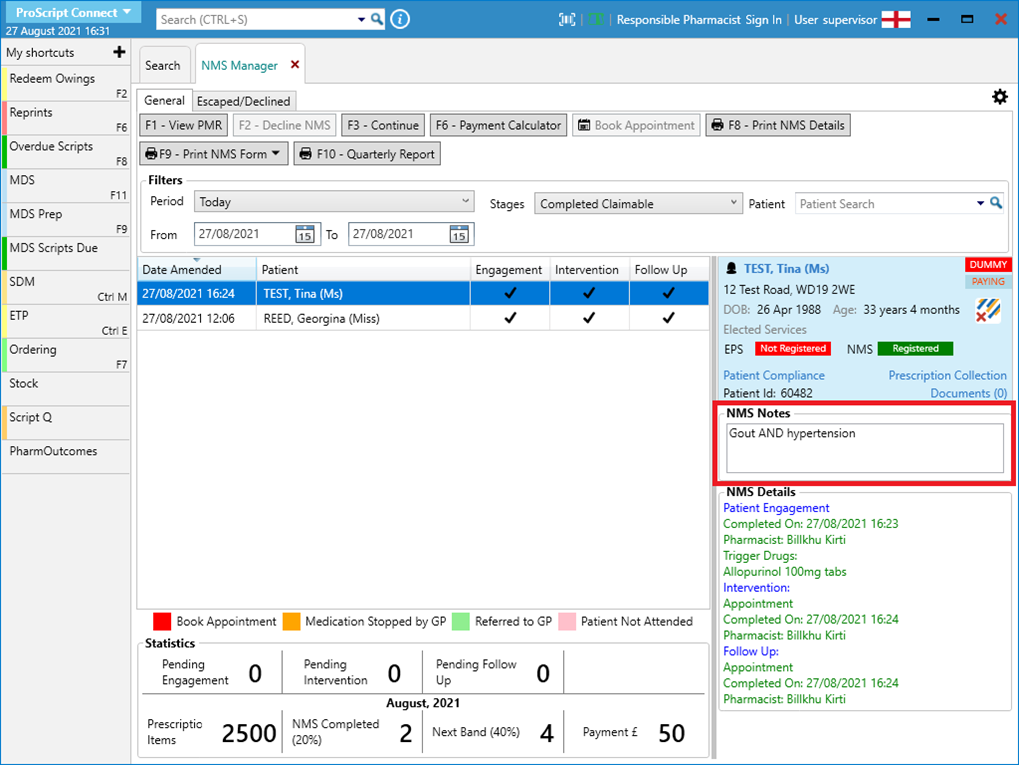
- Navigate back to the NMS Manager and for each patient, check the notes you have added and make a note of this detail:
- When it comes to submitting the Quarterly report, output it to Excel and open:
- You will need to scroll across to the “No. pts in each group*” section (this will be in blue).
- Insert a new column for every new condition you have completed the NMS for.
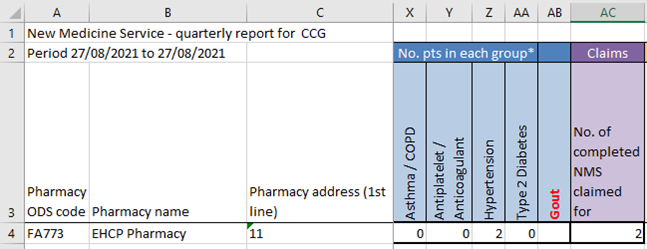
- For any conditions that you have entered for an NMS that were actually for another condition, deduct the figure from the incorrect group and add it to the new group.
- If you have recorded any NMS under the antiplatelet/anticoagulant group that actually fall under one of the new therapy areas, then you will only need to deduct from this group:
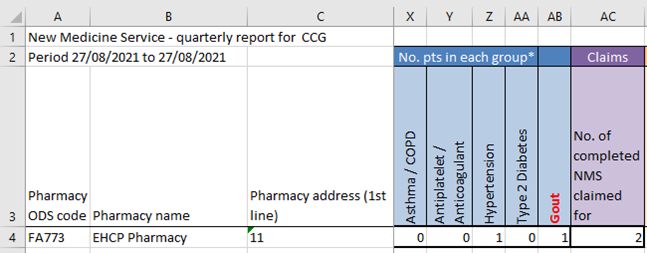
- For any conditions that you have entered for an NMS that were actually for multiple conditions, simply add the figures to the new group(s).
- Always ensure that the total number of patient in each group add up to the total number of claims.
Catch-up NMS
- Catch up NMS: A Catch-up NMS has been granted to patients who were prescribed a new medicine during 31st March 2020 to 31st August 2021 but who did not receive the NMS at that time. This includes drugs in the new therapeutic areas, pharmacists can only conduct these from 1st September 2021 to 31st March 2022 (and are included in the 1% cap). We are currently working on a solution that will identify patients who are eligible for a catch up NMS. We will update this page once that is available. Catch up NMS will need to be carried out manually on paper.
Verbal Consent
It has recently been announced that it is no longer a contractual requirement that written consent is obtained from patients prior to the provision of the Flu Vaccination Service, Medicines Use Reviews (MUR), the New Medicine Service (NMS) and Appliance Use Reviews (AUR). Instead, for these services, verbal consent can be obtained and a record of that made in the pharmacy’s clinical record for the service.
In the case of MURs, NMS and AURs, ProScript Connect will continue to ask that written consent has been obtained. This will still need to be ticked however the customer should make a note of the PMR to state that only verbal consent has been obtained.
