We are currently reviewing changes that will be required to the ProScript Connect (PSC) application in response to the Community Pharmacy Contractual Framework (CPFC) Year 4 announcements.
Until these enhancements are implemented, this page will help assist pharmacy contractors in England to meet the requirements of the CPCF through PSC.
Further information can be found on the PSNC website.
Respiratory Domain
Personalised asthma action plans and promoting spacer device use in children prescribed pressurised metered dose inhalers (pMDIs)
The guidance below outlines the recommendations for generating the reports required in ProScript Connect:
- From the ProScript Connect menu, selecting Report Builder.
- From the Report Builder, select the SYSTEM report named QPS: Asthma Referral Report for Children Aged 5-15 Years.
- Select the [F10 – Run Selected Report] button to open the report in a new tab.
- The filters are automatically set for the previous 6 months and can be adjusted if required.
- Export the report to Excel.
Note that this report will only check for a spacer based on the time period selected. If the spacer device is not present in the PMR history within that period of time, the report will show that the patient has not had a spacer device and will appear with a No Spacer Device tag. We recommend that users check the full PMR history – or with the patient – to confirm if a spacer device has previously been prescribed.
To identify patients 5 years and above with asthma to ensure they have a personalised asthma action plan:
- From the ProScript Connect menu access the Patient/Drug Use Report (PDUR).
- Set the date filter for the month the data is collected.
- Tick Patient Demographics and select the Human radio button.
- Select Conditions.
- Select Add and Conditions from the drop down. Search for Asthma then F10- Select
- Then select the following Printing Details:
- Patient Name
- Address / Nursing Home
- Date of Birth
- Export the report to Excel.
Please note this will display all patients that have asthma added as a condition to the Condition & Allergies section of the Patient Details. Alternatively, Prescriptions Reports can be used:
- From the ProScript Connect menu access Prescription Reports.
- Set the date filter for the month the data is collected.
- In Prescriber Item add asthma drug name, e.g. Salbutamol.
- F2 – Export to Excel.
- In Excel, you can use the features to remove patients that appear more than once.
- The report would then have to be ran again, selecting a different asthma drug to identify more patients.
The Asthma Referral Report (found in the Report Builder) can be used to deliver the ‘Referrals for patients using three or more short-acting bronchodilator inhalers without any corticosteroid inhaler in six months’ criteria.
The report will display patients using more than six short-acting bronchodilator inhalers without any corticosteroid inhaler in six months. The report will be updated in due course.
Return of unwanted and used inhalers criterion
Part of this criterion involves the pharmacy declaration of the total number of conversations had with patients and/or their carer or representatives on the safe and environmentally friendly disposal of their inhaler.
Users will be able to add the following record to the PMR to record that a conversation took place with the patient.
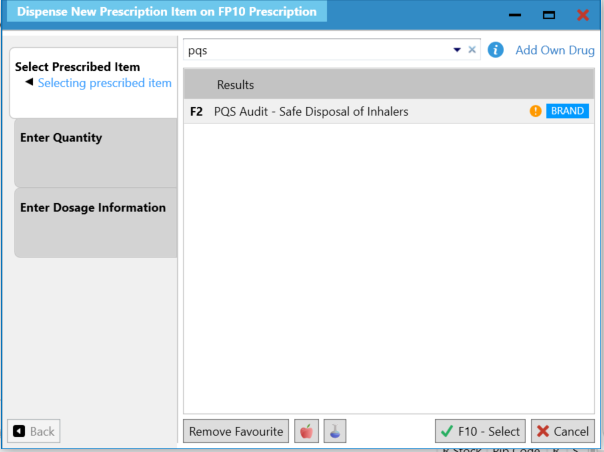
Users can then run the Patient/Drug Use report to identify these patients. In the report, tick the Drug Details and Prescribed check, then select F6-Add. Select PQS Audit – Safe Disposal of Inhalers then F10-Run Report.
